Crop Protection
Pest Management
Aphids,Pentalonia nigronervosa f. typica
Nature of Damage
-
The nymphs and adults congregate under the outer base of the pseudostem
-
Aphids always accompanied by ants, which act as dispersing agents of nymph
-
Honey dew secretion appears on the plants which attracts the ants
-
The aphids suck the sap of the plant and reduce the growth and vigour
-
They also act as a vector of Banana Bunchy Top Virus (BBTV)
|

|
 |
 |
| Aphids present on the leaves |
Bunchy appearance of leaves |
Honey dew secretion |
Identification of Pest
Nymph : Oval or slightly elongated, reddish brown with six segmented antennae
Adult : Small to medium sized aphids, shiny, reddish to dark brown or almost black. They have six segmented antennae and prominent dark veins
Adults start producing young one day after reaching maturity. They can give birth to 4 aphids per day with an average production of 14 offspring per female. |

|
 |
|
Adult
|
Nymph |
Control Measures
Cultural Control
-
Ensure clean cultivation
-
Use healthy and pest free suckers co check the pest incicence
-
Rogue out the affected plants
-
Ratoon and inter crops shoul not be taken up
-
Collect planting material from healthy plants
-
Immersing flowers and foliage in hot water at 49 degrees centigrade for 10 minutes kills banana aphids.
-
Destruction of weeds and alternate hosts.
Chemical Control
-
Spray soapy water or insecticidal soap on plants thoroughly on petioles, furled leaves, whorls or on young suckers
-
Spray Dimethoate (75ml/100lit) or Diazinon (1.5ml/lit) or Acephate (1.3g/lit) on infested plants and suckers
-
Spray Methyl Demeton 25 EC 0.05%
Biological Control
-
Introduction braconid wasps, Lysiphlebius testaceipes as parasitoid to parasitize the aphids
-
Release predators such as lady bird beetles and lace wings in the field which are very active aphid feeders
-
Apply bio control agents like entomopathognic fungus, Beauveria bassiana in the banana fields
|
 |
|
Lady bird beetle
|
 |
| Spraying oil + soap water mix |
 |
| Use healthy suckers for planting |
 Top of page
Top of page
Corm Weevil,Cosmopolites sordidus
Nature of damage
-
Newly planted banana fields are easily susceptible to infestation
-
Infestation begins at the base of the outermost leaf-sheath and in injured tissues at the lower part of the pseudostem.
-
Initially the young grubs make several longitudinal tunnels in the surface tissue until they are able to penetrate to adjacent inner leaf-sheaths
-
Then they bore into the pseudostem base and rhizome/corm, but also into the base of suckers and into roots.
-
Larval tunnels may run for the entire length of fallen pseudostems.
-
Infested plant shows yellowing and withering of leaves, slowed plant growth, root destruction, reduced fruit production
-
Young infested suckers often wither and fail to develop.
-
Plants are easily blown down by mild to strong winds.
-
An economic threshold of 3 weevils per cut banana corm or pseudostem placed in the field overnight will trigger control action
|

|
 |
 |
| Reduced fruit production |
Tunneling and rottening of corms |
Young suckers fail to develop |
Identification of Pest
Egg: elongate-oval, about 2 to 3 mm long and white in colour.
Eggs are laid singly in small cavities that are chewed out by the female in the base of the pseudostem just above ground level, in the upper part of the corm, in roots near the soil surface and at the end of cut stems (stumps).
Egg period is 4 to 36 days depending on temperature conditions.
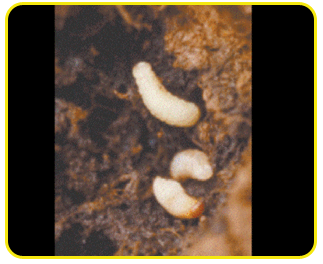
Grub: creamy white legless grubs, stout and distinctly curved and swollen in the middle of the body.
The head is reddish-brown with strong mouthparts. Fully-grown grubs are about 12 mm long.
Grub period is 20 to 25 days
Pupa: white and about 12 mm long. Pupation takes place in holes bored by the grubs. Pupal period is 5 to 7 days
Adult: 10 to 16 mm long weevils, hard-shelled, with a rather long curved snout. Newly emerged weevils are red brown, turning almost black after a few days.
They are free living, they are most commonly found between leaf sheaths, in the soil at the base of the mat or associated with crop residues.
Weevils may live for up to two years, and can live without food for six months, but are very sensitive to desiccation and will die within 48 hours if kept in a dry substrate. They are active at night.
|
|
 |
| Larva |
Pupa |
Control Measures
Cultural Control
-
Field sanitation
-
Use clean planting material – This can be done by selecting vigourous healthy planting material
-
Trimming the suckers
-
Hot water treatment of corms at 52 to 55°C for 15 to 27 minutes
-
Suckers should be pruned periodically and infested pseudostems must be removed from the field and destroyed.
- Banana stumps kept in the field after harvest must be removed and destroyed as they serve as weevil refuges and breeding sites
-
Crop rotation with non host crops like paddy and sugarcane
-
Use mechanical barriers in the field
-
Ensure proper fertilization and free from weeds at all times
-
Use mulch away from the banana stool leaving a clear ring about 60 cm from the base of the stool to keep the roots growing towards the surface and to avoid moist conditions near the stool, which will attract banana weevils
-
Do not take regular crop in the same field to avoid initial infestation
-
Removal of pseudo stems below ground level
-
Avoid growing Robusta, Karpooruvally, Malbhog, Champa and Adukkar
-
Grow less susceptible varieties like Poovan, Kadali, Kunnan, Poomkalli
Chemical Control
-
Cut the banana plant after harvest at the ground level and treat it with carbaryl (1g/liter) or chlorpyriphos (2.5 ml/lit) at the cut surface.
-
Application of Furadan 3G @ 20 gms or Phorate 10G @ 12 gms or Neem cake @ 1/2 Kg. per pit at planting.
-
Before planting, the suckers should be dipped in 0.1 per cent quinalphos emulsion.
-
Remove the pseudostem after harvest and treat it with Carboryl (1g/lit) or Chlorpyriphos (2.5ml/lit).
-
Fumigation of banana plants using
Celphos (aluminium phosphide tablets), especially during
the vegetative phase is phytotoxic and should be discouraged.
-
Apply castor cake 250g or carbaryl 50g dust or phorate 10g per pit before planting also prevents infestation
-
Severe attack dimethoate, methyl demeton, or phosphamidon may be sprayed around the collar region.
Biological Control
-
Natural enemies - Predatory ants such as the bigheaded ant (Pheidole megacephala) and Tetramorium spp feed the eggs, grubs and pupae of weevils.
-
Apply biocontrol agents like Beauveria bassiana and Metarhizium anisopliae,an entomopathogenic fungus, in the banana fields and it causes more than 90% mortality of the weevils
-
Steinerma and Heterorhabditis sp of nematodes attack both adult and grubs in the field.
-
Application of 60 to 100 g of neem seed powder or neem cake at planting and then at four months intervals significantly diminished pest damage and increased yields. Application of over 100 g or neem oil was phytotoxic (harmful to plants) and uneconomical.
Mechanical Control
-
Use of pheromone trap @ 25 traps /ha to destroy the weevil populations.
-
Disc-on-stump traps can be used for trapping weevils. Disc-on-stump traps consist of corm slices placed on top of harvested plants cut at the rhizome. Adult weevils are attracted to the cut stems or corms for shelter, to feed and to lay eggs. The weevils can be collected by hand and destroyed. The efficiency of the traps depends on their numbers and frequency of trapping.
-
Keep the longitudinal split banana traps @ 100/ha.
|

|
 |
 |
|
| Corm injection in banana |
Pheromone trap |
Predatory ants |
 Top of page
Top of page
Nature of Damage:
-
Nematode causes reddish-brown to black,elongated lesions which are readily seen when the roots are split open. Roots eventually blacken and die.
-
Attacks by nematodes, combined with the effects of secondary rot organisms, destroy or weaken much of the root system.
-
Infested plants lack vigour and fruiting is poor.
-
Such plants are readily blown over and the roots are exposed.
|
 |
| Marginal yellowing of leaves |
 |
| Affected palnts topple over easily |
 |
| Drying of boot leaf in advance stage |
Identification of pathogen
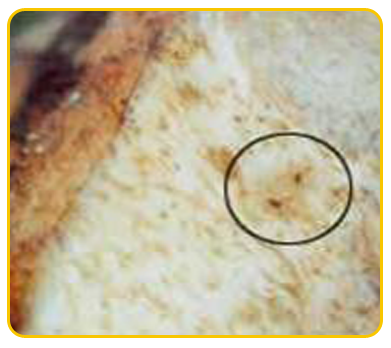
The causal organism of nematode is Burrowing nematode (Radopholus similis), Root lesion nematode (Pratylenchus coffeae), Root knot nematode ( Meloidogyne incognita), Sprial nematode(Helicotylenchus multicinctus)
Meloidogyne spp. Are sedentary endoparasites. Second stage juveniles emerge from the eggs, move towards the roots and penetrate the roots either at the root tip or in regions of previous penetration or where minor wounds are present.The eggs are laid within a gelatinous matrix to form an external egg sac or egg mass. A single sac contains several hundred eggs. Complete life cycle within four to six weeks
Burrowing nematode – eggs are laid in the corms and roots.2 weeks are required for the life cycle to be completed. Female nematodes live 2 to 3 months and lay more than 100 eggs each. Nematode survive in the soil in the absence of suitable hosts
Lesion nematode – Their life cycle takes less than 20 days when temperatures range from 25 degree C – 30 degree C
Spiral Nematodes are robust with strong stylets. They live partially or completely within roots, feeding on the outer cortical cells. |

|
 |
 |
| Meloidogyne incognita |
Nematode damaged corm |
Heterodera oryzicola |
Control Measures:
Cultural Control
-
The use of nematode-free planting material on uninfested land
-
Trim the corm tissue until all black or discolored spots have been removed, leaving only clean white tissues
-
Wash corms in running water, and allow them to dry before planting
-
Submerge trimmed suckers for 20-25 minutes in hot water at 53-54°C.
-
Grow nematode resistant varieties
-
The planting tools should be cleaned before being used in the field
-
Well decomposed manure should be used
-
Crop rotation with non host crops
-
Grow marigold in the inter space which serves as repellent and trap crop
-
Covering the field for 6 to 8 weeks with plastic after tilling and watering raises the soil temperature
Chemical Control
|
|
 |
 |
| Trichoderma-viride |
Nimbecidine |
Banana intercrop with sunhemp |
 Top of page
Top of page
Nature of Damage
-
Infestation of the weevil normally starts in 5 month old plants.
-
Early symptoms of the infestation are the presence of small pinhead-sized holes on the stem
-
Fibrous extrusions from bases of leaf petioles
-
Adult weevils and exudation of a gummy substance from the holes on the pseudostem.
-
During the advanced stages of infestation, when split open the stem, exhibits extensive tunnelling both in the leaf sheath and in the pseudostem
-
Rotting occurs due to secondary infection of pathogens and a foul odour is emitted.
-
When the true stem and peduncle are tunnelled after flowering, the fruits do not develop properly, presenting a dehydrated condition with premature ripening of the bunch itself.
|
 |
| Small pin holes in pseudostem |
 |
| Blackened mass coming out from pseudostem |
 |
| Gummy exudation |
Identification of Pest
Egg: Eggs are cream in colour and cylindrical in shape with rounded ends. The incubation period ranges from 3 to 8 days.
Grub:
The emerging larvae are fleshy, yellowish white and apodous. The larvae feed on tissues of the succulent sheath by tunnelling extensively and may reach as far as the true stem
Pupa:
Pupate in cocoon by winding short pieces of fibrous materials of the sheath around its body. The pupa is exarate and present inside the cocoon.
Adult:
The adult weevils are black-coloured and measure 23-39 mm. They often confine themselves within the pseudostem and in the decomposing tissues of harvested pseudostems. It has a long life span and many adults live for a year.
|
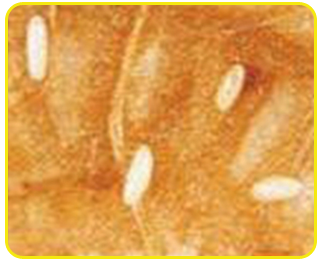 |
| Eggs |
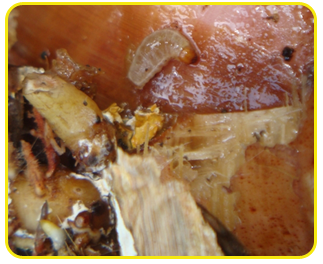 |
| Larva |
 |
| Adult |
Control Measures
Cultural Control
-
Uproot and burn infested plants.
-
Planting material should be trimmed to reduce the number of eggs and grubs.
-
After harvesting the bunch remove and destroy the pseudostem from ground level so as to avoid it serving as a breeding site for the pest.
-
Avoid mattacking (leaving the plant after bunch harvest for recycling of nutrients) in weevil endemic areas.
-
Prune the side suckers every month
-
Use healthy and pest free suckers to check the incidence
-
Do not dump infested materials into manure pit
- Apply mud slurry mixed with neem oil 5% on the pseudostem five month after planting in heavily infested areas to prevent oviposition
- Closely monitor the plants for the detection of oviposition punctureszxzsxz
Chemical Control
-
Application of Furadan 3G @ 20 gms or Phorate 10g @ 12 gms or neem cake @ 1/2 Kg. per pit at planting.
-
Treat the cut end of the leaf petiole with Chlorpyriphos (2.5ml/lit) + 1 ml sticking agent
-
After harvesting of banana bunch cut the tree at base and treat it with 100ml Carbaryl (2g/lit) or apply 10g Beauveria bassiana
Biological Control
-
Swab the cut surface of the longitudinal split traps with 20g of Beauveria bassiana fungus or Heterorhabditis indica nematode and the weevils die on their own due to infection
-
Predatory ants such as big headed any and Tetramonrium spp. are important predators of the banana weevil.
-
Dipping the suckers in 20% neem seed solution at planting
-
Steinerma and Heterohabditis spp. attack both adults and grubs in the field.
Mechanical Control
-
Use Longitudinal Split Pseudostem Traps (LPST) – This traps (45cm long) are made from the pseudostem pieces cut longitudinally in two halves. Such traps are laid randomly in the field @ 25 traps per acre.
-
Disc -on-stump traps and old pseudostems can be used for trapping weevils. Disc-on-stump traps consist of corn slices placed on top of harvested plants cut at the rhizome.
|

|
 |
 |
| Burning of infested Plants |
Stem injection with Moncrotophos |
Pseudostem Trap |
 Top of page
Top of page
Nature of damage:
-
The early symptoms appear as water-soaked smoky areas where the colonies congregate to feed and oviposit between touching or adjacent fruit. These areas then develop the typical rusty-red to dark brown-black discolouration.
-
Further rusty growth of the fruit and yellowing of leaves.
-
While the taste and texture of the fruit within these peels remains unaffected, the exterior discoloration reduces the marketability of affected fruit.
-
In severe cases the skin develops longitudinal cracks and sometimes fruit may split
|

|
 |
 |
| Fruit cracking or spliting |
Rusty red discolouration patches |
Yellowing of leaves |
Identification of pest:
Egg: Eggs are not visible to the naked eye. Eggs are laid just below the fruit or pseudostem surface. In summer, eggs hatch in about eight days
Larva: The wingless creamy white larvae are smaller but have the same shape as the adult. The larval period lasts about 8-10 days.
Pupa: Pupae are white, 1 mm in length, similar to the larvae and can crawl. The pupal stage lasts 7-10 days
Adult: The adult is slender, 1.5 mm long, creamy yellow to golden brown with delicate feathery wings. The front margin of the wings is made up of a fringe of black hairs and, when at rest, these give the adult thrips a characteristic longitudinal black stripe down the middle of the abdomen. Two eye-like dark patches at the base of the wings are characteristic of adult rust thrips. These patches can be used to distinguish from the smaller males of the banana flower thrips. |

|
 |
 |
| Egg |
Pupa |
Adult |
Management:
Cultural method:
-
Use thrips-free planting material or tissue-cultured bananas and, if possible, hot water treatment prior to planting.
-
Destroy all the volunteer plants and that could act as a source of thrips to spread to other plantings.
-
Bunch covers (which cover the full length of the bunch) do provide some protection if applied very early.
-
Regular checking of fruit under the bunch covers is essential to ensure that damage is not occurring.
Biological method:
Chemical method:
-
Bunches, pseudostem and the suckers should be sprayed chlorpyriphos
-
Soil application with Fipronil and Bifenthrin
|

|
 |
 |
| Lace wing bug |
Fipronil |
Removal of Weeds |
 Top of page
Top of page
Rust thrips
Symptoms of damage
 |
Yellowish spots on leaves |
-
Nymph and adult suck the plant sap and inject toxic saliva in the tissue
-
Leaves with greyish yellow spots
-
Stunted growth.
-
Nymphs and adults presence on the lower surface of the leaves.
Identification of the pest:
 |
| Adult |
-
Nymphs – are yellow colour, occur in under surface
-
Adult – yellow colour with minute fringed wings, seen in under surface of leaves
Management
 |
Bud injection to control thrips |
-
Collect and destroy the damaged leaves, flowers and fruits along with life stages
-
Spraying with dimethoate 30 EC - 850 ml/ha or phosphamidon 85 WSC - 300 ml/ha
- Use yellow sticky trap at 15/ha
 Top of page
Top of page
Castor hairy caterpillar, Pericallia ricini
Symptoms of damage
Identification of the pest
 |
Pericallia ricini |
Management
-
Collect and destroy egg masses and caterpillars
-
Use burning torch to kill the congregating larvae
-
Use light trap to attract and kill the adults
- Spray chlorpyriphos 20 EC or quinalphos 25 EC 2ml/lit
 Top of page
Top of page
Cut worm, Spodoptera litura
Symptoms of damage
 |
| Scrabbing of larvae |
Identification of the pest
 |
 |
| Larva |
Adult |
-
Larva - Pale greenish brown with dark marking.
-
Yellow and purplish spots in the sub marginal areas.
-
Fore wing - Stout moth with wavy white markings on the brown.
-
Hind wings - white having a brown patch along the margin.
Management
-
Hank pick and destroy the caterpillar
-
Collect and destroy the damaged plant parts
-
Summer ploughing to expose to the pupae
-
Use light trap 1/ha
-
Severe infestation – spot application of Bt
-
Field release of egg parasitoid
-
Telenomus spodopterae
-
Telenomus remus
- Field release of entomopathogenic fungus, Nomuraea rileyi
 Top of page
Top of page
Banana beetle (Nodostoma subcostatum )
The adult beetles feed on the tender leaves and fruits and remain hidden under unfolded leaves. The infested fruits get spotted and their flavour is affected.
Control
 Top of page
Top of page
Spiraling whitefly (Aleurodicus dispersus)
Spiraling whitefly adults are small (2.0mm long), white and moth-like in appearance and mode of flight. When heavy infestations occur, the adult whitefly and immatures occur in dense populations on the undersides of the leaves of the host plant. These populations are generally covered in a heavy coating of white, curly 'wax' and a sugary secretion that is produced by the whitefly immatures.
Spiraling whitefly is a pest of many horticultural crops, as well as an extensive range of ornamentals and shade trees. It originated in the Caribbean region of Central America, and spread rapidly through the Pacific after gaining establishment in Hawaii in 1978. Not a fly at all, but a relative of the bugs, spiraling whitefly derives its name from the characteristic egg spirals that the adult whitefly lays on foliage and fruit. Without its natural predators it has assumed major pest status.
Symptoms
Damage is mainly caused by the sap-sucking immature and adult whiteflies that feed on the underside of the foliage. Heavily infested plants soon develop a black sooty appearance from mould growing on the sugary secretions that the whitefly immatures excrete. This in combination with leaf damage reduces the plant's ability to photosynthesise and results in loss of plant production.Whiteflies can multiply at a great rate, producing thousands of individuals on a single plant, when natural biological agents are not present. Very high populations may result in defoliation, loss of production and in severe cases, death of the plant.
Control measures
A biological control agent (a parasitoid) was originally established in Torres Strait in 1992 by DPI&F entomologists from Brisbane. This parasitoid is a small, almost microscopic orange-coloured wasp that is host specific to the pest and has already successfully controlled pest populations in Torres Strait and Cape York Peninsula. Parasitoid stocks that have originated from this first establishment are now being used by DPI&F to successfully control mainland populations of spiraling whitefly, in particular those established in the northern tropics. Insecticidal control is not recommended as overseas experience indicates that spraying with insecticides has little long-term impact on the pest and may exacerbate the pest problem by destroying the biocontrol agents.
 Top of page
Top of page
Hard scale, Aspidiotus destructor
Symptoms of damage
 |
 |
Banana scale |
Banana scale |
Identification of the pest
 |
Adult and crawlers |
-
Nymph - Oval translucent, Yellowish brown with waxy coating.
-
Adult - Female circular, semi transparent and pale brown.
Management
Scales (Coccus hesperidum)
Scales cause damage by sucking the juices from the plants. Heavily infested plants appear unhealthy and produce little new growth. Scales feeding on the undersides of leaves may cause yellow spots to appear on the top sides, and these spots progressively become larger as the scales continue to feed. If the scales are not controlled, leaves will drop prematurely, sometimes killing portions of twigs and branches. Scales also feed on trunks and stems of plants.
Cultural Control
Sampling
Inspect plants closely at weekly intervals, especially plants where scale problems have occurred in the past. Since scale insects may occur on all plant parts, every part of the plant must be checked. Leaves should be examined on both surfaces, and particularly along the midrib of the underside. The use of a 10X hand lens or magnifying glass will aid in their detection.
Be sure plants are free of scales before they are placed in the production area of the nursery. Scales cannot fly; therefore they do not readily infest plants as do most other insects.
Chemical Control
Scales, especially armored scales are very difficult to control when mature. Spray applications should be timed to coincide with the crawler stage which is most susceptible to insectisides. Examine plants for live scales by crushing the wax cover. Dead scales do not fall from plants. Select pesticides that have the least effect upon other non-target organisms. For established infestations, apply a second application in two weeks. Horticultural oils are often effective and relatively safe on beneficial organisms. One or two applications of dormant oils should be applied to suppress established overwintering populations.
 Top of page
Top of page
Fruit and leaf scarring beetle (Colapsis hypochlora)
The beetles feed on the young leaves and skin of young fruits. This insect sometimes live in the heart of the pseudostem within the roll of the central leaf. The beetles are mostly found during rainly season. The infested fruits fetch low market value in case of severe scarring of the fruit skin.
Control
-
Practice clean cultivation by removing the grass weeds from the banana plantations.
-
In case of only serious damage caused by the beetles spray the plants with Aldrex 30 EC @ 0.25% ai or dust with Malathion dust.
 Top of page
Top of page
Banana Scale moth
 |
 |
Adult of Banana Scale Moth |
Larva of Banana Scale Moth |
Nature of Damage
-
Banana scale moth caterpillars feed on young leaves, which show scale like scars and refuse of larvae.
Control
- Spray 0.07%. Malathion insecticide on banana seedlings
 Top of page
Top of page
White Grub
 |
 |
Adult of White Grub |
Larva of White Grub |
White grub or root grub is the immature stage of Scarab beetles popularly known as cock chafers, leaf chafer, chafer beetle, May beetle or June beetle.
Host Plants
-
White Grub is a polyphagus pest and feed on almost all kharif season crops.
-
It is widely seen in groundnut crop and chilies of sandy loam soil.
-
White Grub are mainly observed during rainy season in nursery.
Nature of Damage
-
The white grub and adult feeds on the living roots and later adults feeds on shrubs and the trees like neem, khejri etc. growing near the nursery fields.
-
The young grubs after hatching in the soil headed towards the roots and start feeding on them.
-
Consequent to feeding, the plants show varying degree of yellowing, some get wilted and ultimately die. Such affected plants easily collapse.
Control:
-
Collect and destruct beetles in kerosene mixed water and using light traps / pheromone traps during night hours. Spray 0.0 % Carbaryl on the host plants.
-
Deep ploughing of field is a good practice.
-
Pre-sowing soil treatment with Phorate 10G or Quinalphos 5G or Carbofuran 3G @ 300 gm / gunta and treatment of planting materials with Chlorpyriphos @15-20 ml/kg of sukers.
-
Application of Quinalphos or Chlorpyriphos at 400 ml / gunta with irrigation water in standing banana crop.
-
Bio-control: Pathogenic nematode infecting white grub.
 Top of page
Top of page
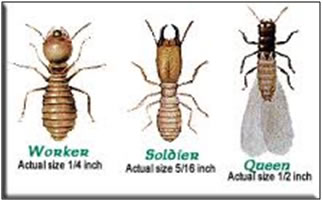 |
 |
Types Termite |
Termite worker |
Host Plants
- Polyphagus pest of all nursery plants.
Nature of Damage
-
In loamy and light soils in dry areas where proper facilities for irrigation are not available, the termite infestation is more serious. The infestation of termite is more in rabbi season.
-
Termites feed on cellulose in the roots of seedling. As a result of infestation, the leaves get dry and seedling can easily be pulled out. In later stage the whole seedling withers.
Control
-
Use of well decomposed organic manure. Remove dead and decaying organic matter or dry stubbles from field to avoid termite infestation.
-
Irrigation protects the plants from termite.
-
Treat soil with Quinalphos 1.5 % or methyl parathion 2 % dust @ 0.25 kg / gunta before planting Suckers.
-
Seed treatment with Chlorpyriphos 20 EC @ 6 ml/kg seed or acephate 75SP @ 4 gm / kg seed.
-
Apply Chlorpyriphos 20EC @ 400 ml / gunta with irrigation water.
 Top of page
Top of page
Nematodes
Burrowing nematode (Radopholus similes)
It is an endo parasite. The nematode enters the roots as well as corms and causes usually induce reddish brown cortical lesions which are characteristic feature of the disease. These lesions are clearly seen when an infected root is split longitudinally. Root and rhizome necrosis is manifested by the retarded growth, leaf yellowing and falling of mature plants. The lesioning of primary roots together with girdling and death of anchoring roots leads the plant prone to ‘tip over’ under wind pressure.
Spiral nematode (Helicotylenchus multicinctus)
This is an ecto as well as endo – parasite. The nematode causes very thin lesion on the roots. The development of feeder roots is affected in case of high population of nematodes in the soil.
Root lesion nematode (Pratylenchus spp.)
These are endoparasites and are very similar to R. similes in habit. In case of severe infestation the cortical region of the root is completely damaged and turns black in colour.
Root knot nematodes (Meloidogyne spp.)
They are endoparasites. They enter the roots remain inside and complete their life cycles. The infected roots exhibit charactesritic swellings and galls at the tips.
Tin general the nematode affected plants exhibit pale yellow leaves with dried margins. In case of severe infestation the plants may fall off on slight push by hand or wind. Planting of infested suckers do not establish and delay growth. Checking of bunches, reduction in total weight and size of fruits are also encountered as a result of heavy infestation.
Control
Paring and pralinage
Paring
The pared sets should be dipped in a Bordeaux mixture – DBCP paste (made by mixing 20 kg hydrated lime, 20 kg copper sulphate, 1288 ml 70% DBCP and 455 litres of water)
Pralinage
The sets should be soaked for a few seconds in 550 ml DBCP plus 40 litres of clay for sets disinfection. This treatment completely coated the set in a persistent nematicidal preparation. In place of DBCP, Carbofuran can also be used @ 40 g / sucker at planting time and another application at the fourth month of crop growth.
-
Follow a suitable crop rotation
-
Flood fallowing for about 5 months destroys not only the Fusarium but also the burrowing nematodes.
-
Application of DBCP @ 40 l / ha at planting time (May and June) and 2.5 l / ha in October gave excellent control of nematodes (Luc and Vilardebo 1961)
-
Application of D-D @ 300 l / ha and EDB @ 150 kg / ha significantly controls the nematodes and increases the yield but is costly.
-
Suckers were trimmed and dipped in slurry solution and sprinkled with carbofuran @ 15 g per sucker.
-
Carbofuran granules @ 20 g should be applied at the time of planting, 2nd and 4th month after planting.
-
Before planting, neem cake and pungam cake @ 200 g should be applied per pit.
-
Sun hemp and chrysanthemum should be grown 45 DAP of Banana and incorporated into the soil one month later.
-
Marigold (Tagetes spp) grown as an intercrop cum trap crop in Banana field resulted in significant reduction of root lesion nematode population and increased the yield.
 Top of page
Top of page
Disease management
Nature of damage
-
The fungus attacks the young banana fruits usually at the distal end
-
At the initial stage, small, circular, black spots develop on the affected fruits. Then these spots enlarge in size, turn to brown colour
-
The skin of the fruit turns black and shrivels and becomes covered with characteristic pink acervuli. Finally the whole finger is affected. Later the disease spreads and affects the whole bunch.
-
The disease results in premature ripening and shriveling of the fruits which are covered with pink spore masses.
-
Occurrence if black lesions on the pedicel causes withering of the pedicel and dropping of the fingers from the hands
-
Sometimes the main stalk of the bunch may become diseased. Infected fruits become black and rotten
|

|
 |
 |
| Dark brown patches on immature fruits |
Fruit turns black and shrivel |
Whole bunch turns black |
Identification of pathogen
-
Acervuli are usually rounded or sometimes elongated, erumpent.
-
Conidiophores are cylindrical, tapered towards the apex, hyaline, septate, branched and sub-hyaline towards the base, each with a single terminal phialidic aperture
-
Conidia are hyaline, aseptate, oval to elliptical or straight cylindrical, obtuse apices or flattened at base and obtuse at the apex, guttulate.
-
The spread of the disease is by air-borne conidia and numerous insects which frequently visit banana flowers also spread the disease
-
The disease is favoured by high atmospheric temperature and humidity, wounds and brusies caused in the fruit and susceptibility of the variety
|
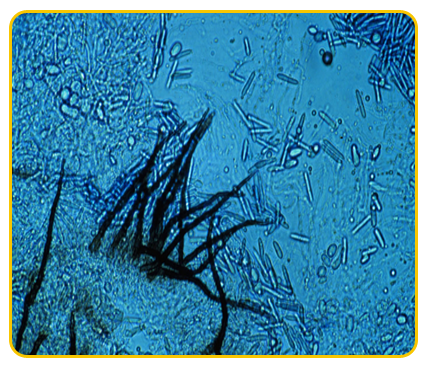 |
| Gloesosporium musarum |
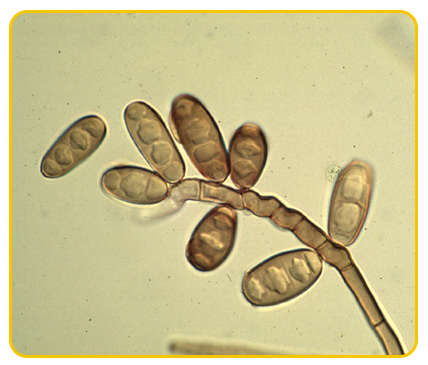 |
| Conidiophores |
Control Measures
Cultural Control
-
Burn the infected materials
-
Proper field sanitation
-
Practice crop rotation with paddy or sugarcane
-
Keep the field free of weeds and provide good drainage
-
Fruit should be free from infection and as possible before it is transported, stored and ripened
-
Banana bunches should be harvested at correct stage of maturity.
-
Proper fertilization prevents the infection
Chemical Control
-
Protective spraying when the fruit is still young with Bordeaux mixture 1%
-
Pre-harvest spray with Prochloroz 0.2% or Carbendazim 0.1% or Chlorothalonil 0.2% four times at fortnightly interval is highly effective
-
Post harvest dipping of fruits in mycostatin 440 ppm or Aureofunginsol 100 ppm or Carbendazim 400 ppm or Benomyl 1000 ppm
Mechanical Control
-
The distal bud should be removed when all the hands opened to prevent infection
-
After harvest, the bunches should be transported to the store house without causing any bruises to them. The transported bunches should be stored carefully at 7 to 10 degree c.
-
Avoid contamination in collecting places, during transport and in ripening rooms
|

|
 |
 |
| Proper fertilization |
Chlorothalonil |
Avoid contamination at collecting place |
 Top of Page
Top of Page
Nature of damage
-
The disease is characterized by the presence of spindle shaped pinkish to reddish streaks on pseudostem, midrib and peduncle
-
Typical mosaic and spindle shaped mild mosaic streaks on bracts, peduncle and fingers also observed
-
Suckers exhibit unusual reddish brown streaks at emergence and separation of leaf sheath from central axis
-
Clustering of leaves at crown with a travelers palm appearance, elongated peduncle and half filled hands are its characteristic symptom
|

|
 |
 |
| Reddish streaks on pseudostem |
Mosaic streak on bracts |
Dark streaks on midrib of leaf |
Identification of pathogen
-
The disease is caused by a virus belonging to potyvirus group. The virions are flexuous filamentous
-
The virus is transmitted through aphid vectors such as Aphis goosypii, Pentolonia nigronervasa and Rhopalosiphum maidis. In field the disease spread mainly through suckers
|
 |
| Aphis gossypii |
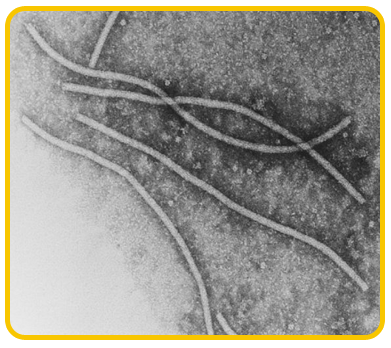 |
| Potyvirus |
Control measures
Cultural Control
-
The diseased plants should be removed as and when noticed to avoid the spread of the disease
-
Disease free planting materials should be used for new planting
-
The banana gardens should be kept free from weeds
-
Weeds in the nearby areas should be removed as the virus survives in them in off-season
-
Early detection by regular inspection of planting and eradication of diseased plants from the field as soon as they are noticed
|
 |
| Selection of healthy suckers |
 |
| Diseased plant should be uprooted |
 |
| Phosphomidon |
 Top of Page
Top of Page
Nature of Damage
-
Initially, dark green streaks appears in the veins of lower portion of the leaf midrib and the leaf stem
-
Dark green, hook-like extensions of the leaf lamina veins can be seen in the narrow, light-green zone between the midrib and the lamina.
-
On mature plants infected with BBTV, new leaves emerge with difficulty, are narrower than normal, are wavy rather than flat, and have yellow (chlorotic) leaf margins.
-
They appear to be “bunched” at the top of the plant, the symptom for which this disease is named.
-
Severely infected banana plants usually will not fruit, but if fruit is produced, the banana hands and fingers are likely to be distorted and twisted.
|
 |
| Bunchy appearance of leaves |
 |
| Bunchy appearance of matured plants |
 |
| Yellowing of leaf margin |
Identification of Pathogen
-
The virus is an isometric particle measure 20nm in diameter.
-
It is ssDNA virus belonging to Nanoviridae family and babu virus genus.
-
The virus has multi component genome
-
There are six circular single stranded genomes known to be established
-
The virus concentration is more present in phloem
-
It is transmitted by infected suckers and banana aphid
|
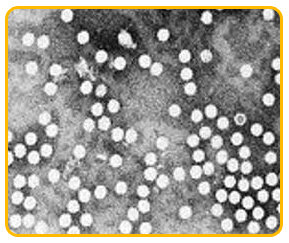 |
| Microscopic view of bunchy top virus |
Control Measures
Cultural Control
-
Use virus free planting materials
-
Remove and rouging of infected banana plants
-
Chop,dry and bury the infected plants
-
Maintain clean, weed free field for early detection of infested suckers
-
Avoid banana cultivation in sugarcane and cucurbitaceous areas as sugarcane mosaic virus or cucurbit mosaic virus can easily spread to banana
Chemical Control
-
The diseased trees should be injected with 4 ml of Fernoxone solution(50g in 400 ml of water)
-
Insertion of Fernoxone capsules (containing 200 to 400 mg of chemical per capsule) into the pseudostem by using the banana injector or capsule applicator
|
 |
 |
 |
|
Virus free planting materials
|
Remove the affected plants |
Capsule application of Feronoxone |
 Top of Page
Top of Page
Nature of Damage
-
The pathogen affects even the immature fruits. The upper portion of the peduncle is exposed to the hot sun, when the bunch emergence occurs during summer months and due to reduced functional leaves reduced
-
The infection, which occurs in perianth, spreads to fingers causing blackening of the skin, shrinkage and folding of the tissues.
-
The affected tissues are corrugated and covered with fungus conidiophores and powdery grey conidia resembling ash on a cigar end.
-
Dry rot also occurs in the pulp and the affected tissues become dry and fibrous.
-
Warm and moist conditions favour the disease occurrence and the disease spread is high in old and badly maintained plantations.
|
 |
| Black necrosis at the tip of fruit |
 |
| Blackening of skin |
 |
| Presence of powdery ash |
Identification of Pathogen
-
Conidiophores are solitary or in small groups.
-
Conidia are hyaline, oblong to cylindrical. They are borne at the ends of tapering phialides, aggregated into rounded, mucilaginous translucent heads.
|
 |
| Verticillium theobromae |
 |
| Conidiophores |
Control Measures
Cultural Control
-
Young bunches should be opened up to the light and air and the bracts which remain attached to the bunch should be removed especially during wet weather
-
The plantations should have enough aeration by avoiding overcrowding of plants
-
Improved sanitation helps in the reduction of the disesase
-
By placing polythene sleeves over the stems before hands emerge
Chemical Control
-
The bunches may be sprayed with Copper oxychloride 0.25 per cent solution along with a wetting agent @ 0.5 to 1.0 ml per litre of spray fluid
-
Spraying of the peduncle with Carbendazim at 0.1% or Dithane M-45 at 0.1% after shoot emergence
Mechanical Method
|

|
 |
 |
| Field sanitation |
Removal of pistal |
Copper oxychloride |
 Top of Page
Top of Page
Nature of Damage
-
This disease is more pronounced on young suckers leading to rotting and emitting of foul odour
-
Roting of collar region is a commonest symptom followed by epinasty of leaves, which dry out suddenly
-
If affected plants are pulled out it comes out from the collar region leaving the corm with their roots in the soil
-
Splitting of pseudostem is common in late stage of infection in cultivars Robusta, Grand Naine and Thella Chakkerakeli
-
When affected plants are cut open at collar region yellowish to reddish ooze is seen
-
In early stage of infection dark brown or yellow water soaked areas are more in the cortex area
-
In advance stage the interiror lesions may decay to such extent that cavities surrounded by dark spongy tissues are formed
-
This soft rotting may spread radially towards growing point through the cortical tissues. The rotten corm emits foul smell
|
 |
| Rotting of collar region |
 |
| spilliting of pseudostem |
 |
| sudden drying of leaves |
Identification of Pathogen
-
The pathogen is a Gram-negative bacterium with peritrichous flagella and it is a rod shaped bacterium that lives alone or aggregates into pairs and chains
-
The pathogen is soil borne and enters through wounds and also through leaf sheath of suckers
-
The disease can be spread by infected plant debris, plant wounds and injuries. Hot and damp weather with plenty of rainfall trigger the disease to occur. Water is required for the bacteria to invade into the plant
|
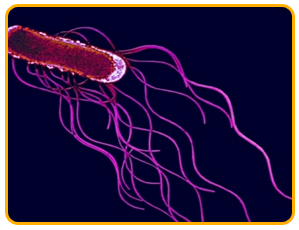 |
| peritrichous flagella |
Control Measures
Cultural Control
-
Good drainage and soil conditioning can control the disease to some extent.
-
Plant disease free suckers.
-
Remove infected plants immediately.
-
Remove plant residues after harvest.
-
Practice crop rotation by using crops that are not susceptible to the bacterial rot disease like soyabean, forage legumes and small grains. Banana should not be grown along with onion and other vegetables.
-
Control nematodes and other insect pests that serve as vectors of the bacteria to invade the plant tissues.
-
Use of rhizomes with dead central buds and active lateral buds prevents the appearance of the disease.
-
Avoid planting during rainy season and use of bigger suckers (more than 500g) for planting.
Chemical Control
-
Drench with Methoxy Ethyl Mercuric Chloride (Emisan-6) 0.1 / or Sodium hypochlorite 10% or Bleaching powder 20g /litre/tree.
-
Drench suckers 2% bleaching powder to control the disease in field at planting followed by another drenching the soil after 3rd month of planting to control the pathogen.
|

|
 |
 |
| Drench suckers with bleaching powder |
use healthy sucker |
Good drainage & well soil condition |
 Top of Page
Top of Page
Nature of damage
-
The disease is characterized by the presence of typical mosaic-like or discontinuous linear streaking in bands extending from margin to midrib.
-
Rolling of leaf margins, twisting and bunching of leaves at the crown and a rigid erectness in newly emerged leaves
-
The presence of dead or drying suckers is noticed in advanced cases referred as heart rot resulting from rotting of heart leaf and central portion of pseudostem
-
Primarily infected banana plants develop severe mosaic symptoms in young growth showing broadly streaked chlorotic or yellowish green bands and patches or chlorotic mottling distributed in patches over the leaf lamina
-
The leaves are narrower and smaller than normal and the infected plants are dwarf and lag behind in growth. Such plants do not produce bunches but as a virus reservoir
|
 |
| Mosaic appearance of leaf |
 |
| Bunch of leaves at crown |
 |
| Heart leaf get rot |
Identification of pathogen
-
The casual agent of this disease is Cucumber mosaic virus(CMV). The virus is isometic, linear positive sense and single stranded RNA. The RNA is surrounded by a protein coat consisting of 32 copies of single structural protein which form isometric particles
-
The primary transmission is through use of infected daughter suckers from diseased palnts and the secondary spread of the disease is through melon aphid, Aphis gossypii and Aphids maidis
|
 |
| Potyvirus |
 |
| Aphis gossypii |
Control Measures
Cultural Control
-
The banana gardens should be kept free from weeds
-
Infected suckers should not be used for planting
-
Weeds in the nearby areas should be removed as the virus survives in them in off-season
-
Growing pumpkin, cucumber and other cucurbits between the rows of banana crop should be avoided
-
Dry heat treatment of suckers at 40 degree C for one day inactivated the virus
-
Early detection by regular inspection of planting and eradication of diseased plants from the field as soon as they are noticed
-
Use of high input crop management of system comprising of 10 kg farm yard manure at the time of planting and subsequently at an interval of 3 months. 1 kg neem cake, 200 gm nitrogen, 40 gm phosphorus, 200 gm potassium per plant, 4 weeding at 2 months interval up to 8 months stage
Chemical Control
-
Spraying Methyl Demeton 0.03 per cent (0.3 ml/lit of water) at 3 to 4 weeks interval controls the vector and reduces the spread of the disease
-
Foliar spray of micronutrient (0.2% CuSo4(2ml/lit), 0.2% FeSo4(2ml/lit), 0.5% ZnSo4(5 ml/lit) and 0.1% H3Bo3(1 ml/lit of water) at 3,5 and 7th month after planting and spraying Glyphosate 2Kg per hectare to control weeds
-
Control of insect vector by spraying Phosphomidon at 1 ml per litre or methyl demeton at 2 ml per litre
|

|
 |
 |
| Demeton |
Weeds free field |
Proper nutrient management |
 Top of Page
Top of Page
Nature of Damage
-
Externally, the first obvious signs of disease in most varieties are wilting and a light yellow colouring of the lower leaves, most prominent around the margins. They eventually turn a bright yellow colour with dead leaf margins.
-
In the advanced stages of disease, affected plants may have a spiky appearance due to prominent upright apical leaves in contrast to the skirt of dead lower leaves.
-
Internally, symptoms first become obvious in the xylem (water conducting) vessels of the roots and the rhizome. These turn a reddish-brown to maroon colour as the fungus grows through the tissues.
-
When a cross-section is cut, the discolouration appears in a circular pattern around the centre of the rhizome where the infection concentrates due to the arrangement of the vessels. As symptoms progress into the pseudo-stem, continuous lines of discolouration are evident when the plant is cut longitudinally
|
 |
| Yellowing and withering of leaves |
 |
| Complete withering of leaves |
 |
| Vascular discoloration in pseudostem |
Identification of Pathogen
-
The casual organism is Fusarium oxysporum f.sp.cubense.
-
This pathogen contains colonies of white to purple pigemented mycelium. Hyphae are septate and hyaline. Conidiaphores are short and simple and having macroconidia and microconidia
-
Macroconidia usually produced abundantly, slightly sickle-shaped, thin-walled, with an attenuated apical cell and a foot-shaped basal cell. They are three to 5-septate measuring 23-54 x 3-4.5 µm.
-
Microconidia are abundant, mostly non-septate, ellipsoidal to cylindrical, slightly curved or straight, 5-12 x 2.3-3.5 µm occurring in false heads from short monophialides.
-
The disease is soil borne and the fungus enters the roots through the fine laterals. The disease incidence
-
The incidence is high in acid alluvial soils.
-
The pathogen is easily spread by infected rhizomes or suckers, farm implements or vehicles, irrigation water
|
 |
| Fusarium oxysporium in situ |
 |
| Fusarium oxysporium - Macroconida |
 |
| Fusarium oxysporium – Microcoinidia |
Control Measures
Cultural Control
-
Practice proper crop rotation with paddy/sugarcane once or twice followed by banana for 2-3 cylces
-
Plant wilt resistant cultivars such as Poovan and Nendran in endemic areas
-
Avoid susceptible varieties such as Rasthali, Monthan, Karpuravalli, Kadali, Rasakadali, Pachanadan etc.
-
Remove and destroy infested plant material after harvest
-
When only 1-3 plants are infected, kill and chop up the diseased plants and stew all the material in water at a temperature of at least 70 deg C for 30 minutes.
-
Grow healthy plants with proper fertilization, irrigation, weed control
-
Provide good drainage especially during rainy season
-
Soil application of rice chaffy grain or dried banana leaf formulation or well decomposed compost around the plants
Chemical Control
-
Application of 2 per cent of Carbendazim as injection of Carbendazim 50 ml capsule application
-
Paring (pralinge removal of roots and outer skin of corm) and dipping of the suckers in a solution containing 0.2 per cent Carbendazim + 14 ml of per litre of water. Instead of Monoctophos the suckers may be dipped in clay slurry and sprinkled with Carbofuran granules at 40g/corm
-
Soil drenching of Carbendazim 0.2 per cent solution alternated with Propiconozole 0.1% around the pseudostem at bimonthly intervals starting from five months after planting
-
Application of urea + sugarcane trash (250g/pit) followed by lime (1Kg/pit) and neem cake (1-2Kg/pit)
-
Application of neem cake @ 250 Kg/ha was most effecgtive in controlling Fusarium wilt in Rasthali cultivar
Biological Control
-
Application Pseudomonas fluorescens a bactericide can also be applied along with farmyard manure and neem cake.
-
About 60 mg of Pseudomonas (in a capsule) can be applied in a 10 cm deep hole made in the corm.
-
Application of bio control agents like Trichoderma viride @ 25 g for 4 times once at the time of planting in the planting pit and remaining doses at third, fifth and seventh month after planting
-
Application of T.harzianum Th-10, as dried banana leaf formulation @ 10g/platn in basal + top dressing on 2,4,and 6 months after planting
-
Dipping the planting materials in spore suspension of P.fluorescens at 10g per plant at 3,5 and 7 months after planting
Mechanical Control
-
Machinery and equipment should be treated with a sanitary solution such as Farmcleanse®
-
Footwear, which may have contacted banana plants or soil around banana plants elsewhere, should not be worn on the farm.
-
No agricultural vehicles, tools (including shovels, knives and ladders) or equipment should be removed from, or brought on to, the farm without prior approval from management.
-
Provide mechanical barriers in and around the infected plants
|

|
 |
| Crop rotation with paddy |
Burning of wastes |
 Top of Page
Top of Page
Nature of Damage
-
Initially, appearance of pale yellow or greenish streaks parallel to the veins on the upper surface of the leaves
-
Then these streaks darken and become more or less elliptical brown spots.
-
Later on, the centre of these spots turns to light grey colour surrounded by yellow halo
-
The spots often coalesce to form large irregular patches of dried tissue
-
Rapid drying and defoliation of leaves are the characteristic feature of this disease
-
Normally 15-18 leaves are necessary at the time of shooting for bunch development, but due to Sigatoka leaf spot it is difficult to maintain 15 leaves
-
In severe cases, immature bunches fail to fill out
-
The fingers of bunch in affected plants tend to remain undersized and angular but pulp starts ripening
|
 |
| Pale yellow streaks on the upper surface of leaves |
 |
| Centre of the spots turns to grey colour |
 |
| Rapid drying and defoliation of leaves |
Identification of Pathogen
-
This disease is caused by Mycospharella musicola fungus by the characteristics of the conidia and conidiophores
-
The conidiophores are bottle shaped and bear conidia. Conidia are narrow and multiseptate
-
Perithecia are dark brown to balck, amphiceous, erumpent, ostiolate
-
Asci are oblong, clavate. Ascopores are one septate, hyaline, obtuse-ellipsoid with upper cell slightly broader
-
The conidia of the fungus are carried by wind ,rain water and old dried infected leaves and they help to spread the disease
|
 |
| Microscopic view of Mycospharella musicola |
Control Measures
Cultural Control
-
Removal and destruction of affected leaves.
-
Keep the banana field as weed free and remove the suckers timely.
-
Avoid planting at close spacing.
-
Provide proper drainage and avoid water logging in the fields which favours infection.
Chemical Control
-
Spray Bordeaux mixture 1 per cent + linseed oil 2 per cent on the plants.
-
Spray Copper oxychloride or Zineb with gas oil or mobile oil or white oil.
-
Spray 3 times with Carbendazim 0.1 per cent or Propicanozole 0.1 % or Mancozeb 0.25% or Calixin 0.1% and teepol (sticking agent) at 10-15 days interval, as the disease starting from initial appearance of leaf specks in lower side of the leaf.
|

|
 |
 |
| Removal of affected leaves |
Weed free field |
Mancozeb fungicide |
 Top of Page
Top of Page
Non Insect Pests
Giant African Snails (Achatina fulica Bowtich)
Symptoms
 |
 |
 |
| African snails Banana leaves holes |
Feeds the leaves and make holes |
Snails feeding banana pseudostem and petiole |
-
External feeding on foliage and fruits.
-
Affected plant stages - .Seedling stage, vegetative stage and fruiting stage.
-
Affected plant parts -
Leaves and fruits.
-
Infest 41 locations in four districts in Kerala state.
-
Palakkad has been identified as the most vulnerable district with the study predicting attack in 22 locations. Puthunagaram, Kodumbu, Peruvembu, Mundur, Puthuppariyaram, Marutha Road, Kannadi, Koduvayur, Chittur-Thathamangalam, Mathur, Pirayiri, Vadavannur and Thenkurissi are some of the vulnerable areas in the district
Identification of pest
 |
 |
 |
Snail Eggs
|
Banana African Snails
|
Banana African Snails
|
-
Giant East African Snail has a narrow, conical shell, which is twice as long as it is wide and contains 7 to 9 whorls when fully grown. The shell is generally reddish-brown in colour with weak yellowish vertical markings
-
Life expectancy is commonly five or six years in captivity, but the snails may live for up to ten years. They are active at night and spend the day buried underground.
-
It is an obligate-outcrossing hermaphrodite, which means that one externally fertilised snail can establish a population .
-
Fulica produces large eggs that are 4.5 to 5.5 mm in diameter and only hatch at temperatures above 15°C. Snails begin laying eggs at six months of age and fecundity lasts approximately 400 days.
-
Their eggs are laid in batches of 100-400 and are spherical to oval in shape, approximately 5 mm in diameter and cream to yellow in colour.
-
Dependent on the temperature, the babies will hatch in anything from 5 to 21 days. Snails mature at around 5 to 15 months, depending on the temperature (with cold winter temperatures inducing hibernation and delaying sexual maturity).
-
The snails, which live up to six years in favourable conditions
-
The infestation was found to be intense in areas with high population density. Areas with untreated garbage and places of water logging are their favourite spots
Control measures
-
Crop rotation, enhancement of soil quality, choice of resistant varieities, water management, providing mechanical barriers, post harvest treatment
-
Practice good field sanitation
-
Use of calcium arsenate and Metaldehyde under expert supervision in areas of high infestation.
-
Use of salt to kill the pest as it alters the soil pH
-
The application of salt will become untenable during rainy days
-
The meat of the snails thus killed will rot with foul smell
-
Release predators like predatory snail (Euglandina rosea), flatworms (Platydemus manokwari) and pathogens in the field
-
Metal salt-based molluscicides as snail baits and snail pellets. These are derived from iron phosphate, copper sulphate and aluminium sulphate. They are not toxic to humans and animals (In organic culture, consult your certification body before use)
Rats (Rattus exulans)
Symptoms
 |
 |
 |
Banana Rat
|
Rat feeding injury on fruits
|
Rats nest on banana bunch
|
Identification of non pest
 |
| Pacific rat D Vietch |
-
Comparatively small in size, weighs 2 to 3 ounces, and measures 4 to 5 inches long. The tail is as long as or slightly longer than head and body combined; bristles along the tail give the appearance of faint, narrow rings.
-
The body color is cinnamon-brown to cinnamon-buff to grey with stiff black guard hairs on back and sides; the underside is light buff or grey.
-
The nose is roundly pointed, ears rather short, eyes medium size, hind feet dark on underside. Females have four pairs of nipples.
-
It nests in burrows, gulches, rock piles, rock walls, wastelands, fields, and embankments. It causes great damage to sugarcane, banana, pineapple, coconuts, coffee, and other fruit and vegetable crops.
Management
-
Remove access to food, water, or shelter, or limit rat accessibility; traps; trim overhanging trees away from coffee plants; cats and dogs.
-
Rodenticides are the most effective means of controlling large and small rodent populations. Strict safety precautions should be used in the preparation, broadcast, or placement and disposal of poison baits for rodents.
Ants (Anoplolepsis longipes)
Symptoms
 |
 |
 |
|
Formic acid damage to young banana bunches and fingers
|
Ant Damage |
Ant Damage |
-
Some ant species associated with banana bunches can cause significant damage to young fruits, especially when colonies of the ants on bunches are disturbed or agitated by bunch spraying or by bumping into plants and causing vibration disturbance to the ants.
-
The disturbed ants eject formic acid from their abdomens as a defense reaction.
-
The formic acid, with a pH of 2-3, burns the tender skins of the young fingers, leaving irregularly shaped, blackened areas on the fruits that expand as the fruits expand.
Identification of insect
 |
| Banana Ants under old banana sheat |
Eggs:
Larvae:
Pupae
Adults:
Control measures
 Top of page
Top of page





















































 Top of page
Top of page
















































































