A. Screening techniques for pest and disease resistance
The different screening techniques for pest resistance are listed below.
1. Field Screening
The varieties to be evaluated or the segregating population to be screened for resistance may be raised in fields under natural infestation, either in endemic areas or by adopting techniques for increasing field infestation. Cultural practices such as closer spacing to create the most desirable humid microclimate within the crop, application of additional dose of nitrogen and irrigation to induce vegetative growth may be adopted. The  test material may be sown or planted early, before the adult emerge in an area already infested in the previous season. test material may be sown or planted early, before the adult emerge in an area already infested in the previous season.
In case of insects such as plant hoppers, flies etc. that have a tendency to move at a rapid speed, fibre glass mesh cages may be kept on microplots and artificially reared insects released at a specified number per plant.
The insects population should be able to infest the plant population uniformly so that plants that escape infestation are not graded as resistant.
Highly susceptible plants can be interplanted as “spreader rows” along with rows of the test material. Insect attractants, such as fish meal for sorghum shoot fly may also be used to attract and increase the insect density in the field.
2. Green house screening
Screening the varieties or segregants in green houses providing conditions conductive for infestation is more rapid reliable than field screening. Special methods have been developed to increase the insect population to provide sufficient insect pressure for valid screening. Insects reared in the culture maintenance cages are released on plants raised in seed boxes kept in green house. Fibre glass screen cages are used for each seed box.
Depending on the crop on one hand and the insect pest on the other, the stage of the crop at which the insect is released, the stage of the insect, whether egg, larva or adult and the number of the insects population vary, as also the symptoms on the host to differential and grade the resistant plants from the susceptible.
3. Laboratory Screening:
Laboratory screening for resistance can also be done using plant tips or leaf discs and allowing forced or free choice feeding by insects as in the case of lucerne weevil.
4. Bioassay techniques
Bioassay techniques are also used to screen resistance to insect such as Heliothis spp and pink boll worm in cotton. Lyophilized square powder is incorporated in an artificial diet and dispensed into two-ounce plastic containers. Late first or early second instar larvae of Heliothis are weighed and placed in the diet cup. By periodic observations, larval survival, larval growth and percent pupation are recorded. Dates on fecundity and longevity of emerging adults are obtained to screen resistant types.
B. Screening Techniques for Diseases Resistance
The first step in a resistance breeding programme is to rapidly screen all the available genetic stocks, including the local land races, improved cuttings and exotic germplasms using empirical techniques in glass houses, or by field tests. An escape from disease is a hazard of breeding for resistant varieties. There are two major techniques to achieve the objectives.
1. Artificial inoculation
The artificial inoculation of test genotypes is necessary to obtain a more uniform disease – epidemic than world occur naturally such. A uniformity facilitates comparison among disease genotypes. For a successful screening, an adequate amount of inoculum is necessary, since selecting for resistance in the presence of an unadequate amount of disease create an instant artifact. Among few methods developed, three main procedures of artificial inoculation are generally employed.
- Spraying the spore suspension on test genotypes – using an autominyor the aqueous suspension of the pathogens propagules is sprayed. High pressure spraying is most productive.
- Injection of spore suspension into the plants surface or into the intercellular air spaces of a leaf with the help of a hypodermic needle. A single or multineedle pricking may be employed.
- Immersion of seedlings of test genotypes in a spore suspension before transplanting them into fields. Since the host tissue becomed water soaked, they predispose themselves to disease.
Though spore – suspension in sterile water (Inoculum) is often used, successful inoculum of many pathogens can be achieved only if dry spores are employed. Spores are damaged quickly if they became too wet. For good results, any one of these three methods of artificial inoculation can be used. Depending upon the convenience and crop materials the procedure may vary from one disease to another and from one crop to another. But similarities are not ruled out.
2. Artificial epiphytotics
The manual operation of artificial inoculation can be dispensed with once a sick plot or disease nursery is created. Artificial creation of such an epiphytotic is necessary because the occurrence of natural disease is subject to chance. This can be attained by seeding spores in the soil (in case of only soil borne disease caused by facultative parasites) either through growing a highly susceptible genotype in a plot for successive years, or through mixing infected debris in the soil of that plot, prior to sowing the test genotypes. Maintenance of requisite humidity is essential for high effectiveness.
1. The epiphytotic should be intense developed at both national and international levels. The international wheat rust nursery at Mexico established since 1957 is an outstanding example of such an international collaboration.
2. Frequency of exposure: The test genotypes can be subjected to artificial inoculation or sick plots once or more than area for desired results. Two tests are suggested.
a. Polycyclictest: Exposure that initiates the natural occurrence of diseases with a recurrent infection cycle attacking a crop on the farmers field. The artificially created sick-nursery is the best form of such polycyclic tests, through repeated artificial inoculations can also achieve the same results.
b. Monocyclictests: Exposure only once by artificial inoculation, preferably under closed/ controlled condition, such as green houses.
However in actual practice neither of the two tests are employed by breeders. A continous monocyclic test is applied since the moderately resistance cultures are subject to the monocyclic test everyday and which is conductive to injection.
Testing the host plants either by artificial inoculation or under sick nurseries is the beginning of the screening process. Next comes how to measure the intensity of diseases.
3. Measurement of the intensity of disease infection.
There are two methods to assess the disease intensity depending upon the inheritance of the disease.
Qualitative Method: If reflects the response type representing a class but not the magnitudes in monogenic disease control.
Quantitative Method: This quantifies or determines the degree of infection in terms of grades or percentages or indices.
The following are commonly used.
Simple usual scoring: Each plant or group of plants may be assigned a numerical score (grade) for the severity of symptoms as under (Russell, Q78)
O = nosymptoms, 1 = very mild symptoms, 2 = mild symptoms, 3 = severe symptoms and 4 = very severe symptoms.
Pate and Harvey (1954) also used scores from 0-5 with O for immunity and 5 for very heavy infection ofHelminthosporium maydis.
Grading system of Harsfall and Barrat (1954): At below 50 percent infection, the eye sees the amount of disease tissues and at above 50 percent infection, it sees the amount of disease free tissues. The simple usual scoring does not take care of this principles. However, Hargfall and Bassats grading systemic based on 50 percent as mid part as under.
Grade |
1 |
= |
0 % Kill |
Grade |
7 |
= |
50-75 % |
" |
2 |
= |
0-31 % |
" |
8 |
= |
75-87 % |
" |
3 |
= |
3-6 % |
“ |
9 |
= |
87-94 % |
" |
4 |
= |
6-12 % |
" |
10 |
= |
94-97 % |
" |
5 |
= |
12-25 % |
" |
11 |
= |
97-100 % |
" |
6 |
= |
25-50 % |
“ |
12 |
= |
100 % |
Meangrade = grade reading ÷ number of reading.
Grade indicates 0 percent disease, while grade 12 means 100 percent disease infection.
Ullstrip (1944) Diagrammatic Standard: 0.5 = Very light infection, one or two restricted lesions on lower leaves.
- = Slight infection a few scattered lesions on lower leaves.
- = Light infection moderate number of lesions on lower leaves.
- = Moderate infection, abundant lesions on lower leaves, a few on middle leaves.
- = Heavy infection, lesions abundant on lower and middle leaves extending to upper leaves.
- = Very heavy infection, lesions abundant on all leaves plants may be prematurely killed.
A rating of (2.0) is generally a satisfactory lead for breeding purposes.
Infection Index of villareal and Lantican (1965):
0 = No infection (N)
1 = Very slight infection (Vs) very few lesions on lower leaves.
2 = Slight infection (5) moderate number of lesions on loweor leaves.
3 = Moderate infection (M); abundant lesions on lower leaves and a few on middle leaves.
4 = Heavy infection (H); lesions abundant on lower and middle leaves, extending to upper leaves.
5 = Very heavy infection (VH); lesions abundant on all leaves, plants may be prematurely killed.
A Modified Infection Index: The All India Millet workers conference held at Gwalior March 1976 recommended a modified version of the above grading system for scoring downy mildew incidence in pearl millet. According to this scheme, each plant is assigned a security scale/ score as under:
1 = No symptom
2 = Symptom on nodal tillers only (green ear to blind tillers (5 % infection)
3 = Symptoms on main tillers (green ear or blind tillers) three or more productive heads (10% infection)
4 = Symptoms on most main tillers so that there are only one or two productive heads (30% infection)
5 = Symptoms on all tillers ; No productive heads (35% infection)
Drought tolerance
If high yielding varieties involved for irrigated situations are screened rigorously under upland conditions, it would ensure adaptability of selected materials to local conditions as well as a high yield potential.
Many practical agonomists and breeders have realized that the best yielding varieties of irrigated rice are not necessarily the best in rainfed, drought prone conditions.
The following characters should be taken into account while screening for drought resistance.
1. Screening for growth – early maturity is preferred.
2. Screening for seedling vigour
3. Screening for height

4. Screening for root characteristic: Root characteristic such as size, morphology, depth, length, density and function are important in maintaining relatively high leaf water potential against evapotranspirational demand. A deep root system is an important component of drought resistant because it enables the plant to export water in the deep soil layers. Of all the attributes, root length density is probably the major operative factor that largely determines the extent to which roots can extract water from the adjacent soil.
5. Screening for leaf rolling: Variations in leaf water potential among cultivars or strains under moisture stress up to 10-13 bars between extreme rice genotypes have been found.

6. Screening for osmotic adjustment: Osmotic adjustment generally considered as the net increase in ultracellular solutes, usually occurs in response to various environmental stresses.
7. Screening for translocation of photosynthesis: Screening for relative ability many genotypes for translocation efficiency as a measure of waterstress tolerance has been suggested.
8. Screening for amino-acid accumulation: Certain amino-acids increase dramatically under stress and screening for them has been suggested as a means of evaluating drought resistance. Observations suggest that proline could provide metabolic advantages within limits of water stress.
9. Screening for stomatal behaviours: Actions of stomata provide important mechanisms to reduce water loss during stress. Higher leaf water potential can be maintained by stomatal closure.
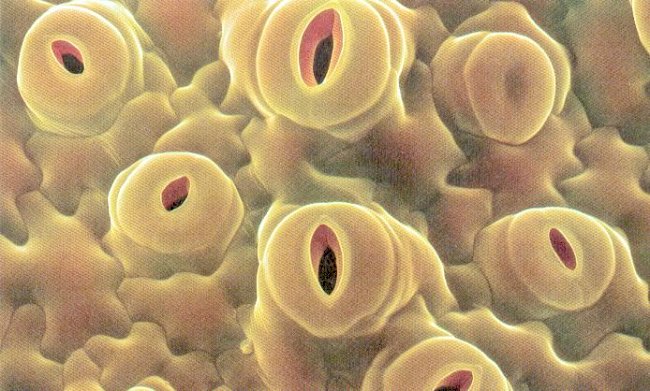
10. Screening for cuticle characteristic: Cuticular permeability or transpiration is affected by thickness of cuticular layer and subsequently is influenced by the amount composition and physical configuration of epicuticular wax deposits.
11. Screening for xylem, vessel structure: Dryland varieties generally have larger xylem vessels in seminal roots than semidwarf. Larger vessels decrease root axial resistance to upward water transport.
12. Screening for desiccation tolerance ability: The development of simple screening techniques based an exposure of leaf portion or seedlings to heat shows wide variation in dehydration tolerance among species and cultivars. |

